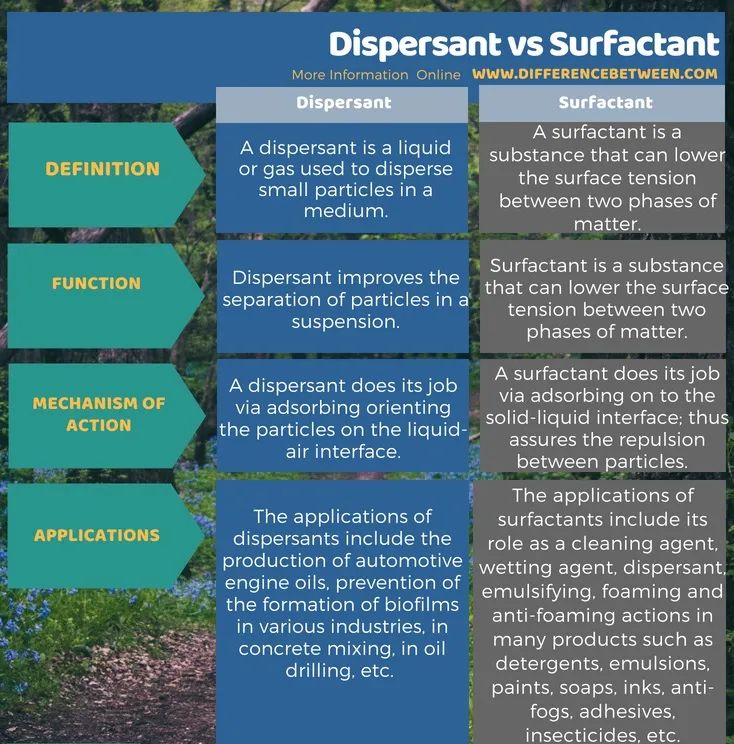
The main difference between dispersants and surfactants is that dispersants improve the separation of particles in a suspension, while surfactants are substances that reduce the surface tension between two phases of a substance.
Dispersants are a form of surfactant. But all surfactants are not dispersants. In addition to acting as dispersants, surfactants can also act as detergents, wetting agents, emulsifiers, and foaming agents. Usually, they are organic compounds.
What is a dispersant?
Dispersants are liquids or gases used to disperse small particles in a medium. We also call it “plasticizer”. They come in two forms. Non-surface-active polymers and surface-active substances. We add these substances to the suspension to avoid the formation of particle clusters. This improves the separation of particles in order to avoid cluster formation. Additionally, this process prevents particles from settling. In most cases, dispersants consist of one or more surfactant substances.
Applications of these substances include the production of automotive engine oils, preventing the formation of biofilms in various industries, avoiding the use of large amounts of water in concrete mixing, and breaking solids into particles in oil drilling.
What is a surfactant?
Surfactants are substances that reduce the surface tension between two phases of a substance. It can reduce the surface tension between two liquids, between a gas and a liquid, or between a liquid and a solid. Most of the time, they are amphiphilic organic compounds. This means that these substances contain both hydrophilic and hydrophobic regions in the same molecule. Therefore, they contain both water-soluble and water-insoluble regions.
Applications of surfactants include their use as detergents, wetting agents, dispersants, emulsifiers, foaming and defoaming in many products such as detergents, lotions, paints, soaps, inks, defoggers, adhesives, sterilizers, etc. Insecticides, etc.
What is the difference between dispersants and surfactants?
Dispersants are liquids or gases used to disperse small particles in a medium. plasticizerare substances that reduce the surface tension between two phases of a substance. However, dispersants are in the form of surfactants. The functions of these two substances are different. This means that dispersants prevent particle clusters from forming in suspension, while surfactants reduce the surface tension between two liquids, between a gas and a liquid, or between a liquid and a solid. This is the main difference between dispersants and surfactants. Furthermore, dispersants work by adsorbing and orienting particles at the liquid-air interface, whereas surfactants work by adsorbing at the solid-liquid interface. Repulsion between particles is thus ensured.